Click-&-Go® Protein Reaction Buffer Kit
Product No. 1262
Introduction
Click-&-Go® Protein Reaction Buffer Kit provides all the necessary reagents to perform copper-catalyzed click reaction with any azide or alkyne tagged protein and the corresponding click detection reagent.The kit includes specially formulated components to both catalyze and protect proteins during the click labeling reaction. Sufficient reagents are provided for 25 labeling reactions containing 50μL protein solution(1-5 mg/mL).
Kit Contents
| Component | Concentration | Amount | Storage | Stability |
|---|---|---|---|---|
| Reaction Buffer (Component A) | 2X | 3.0 mL | 2-8°C | Stable for at least 12 months when stored as directed. |
| Copper (II) Sulfate (Component B) | 40 mM | 300 μL | ||
| Reducing Agent (Component C) | — | 50 mg | ||
| Additive (Component D) | — | 35 mg |
Materials Required but Not Provided
- Alkyne or azide-tagged protein
- Azide or alkyne detection reagents
- High-speed microcentrifuge
- 1.5 ml microfuge tubes
- 1-5 mg/mL azide or alkyne-labeled protein samples
- Solvents: methanol, chloroform, 18 mega-Ohm water
Additional information
- Final concentrations of an azide or alkyne detection reagent may range from 2 μM to 40μM. Final concentrations below or above this range are also possible, and should be optimized per the specific application. We recommend starting with a final concentration of 20μM, and titrating this amount down in case of high background.
- Compatible with protein extraction buffers containing 1% SDS, sodium phosphate buffers containing 1% NP-40, or RIPA Buffer (50 mMTris-HCl, 150 mM sodium chloride).
- Caution-copper (II) sulfate solution is harmful to aquatic organisms and can cause damage to aquatic environments. Avoid release into the environment. Refer to MSDS.
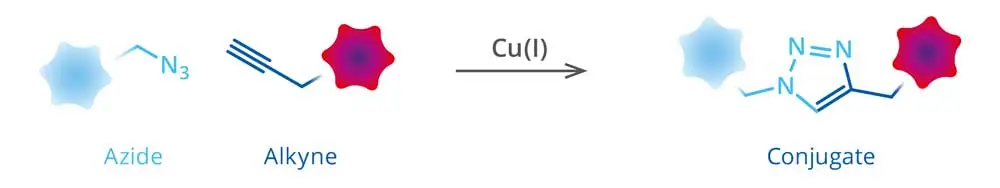
Figure 1. Schematic representation of tagging of an azide-modified protein
with an alkyne-detection reagent via Cu(I)-catalyzed click reaction.
Material Preparation
| Prepare Azide or Alkyne Detection Reagent | Prepare detection reagent of choice in DMSO to a concentration of 2.5-5 mM. After use, store unused detection reagent stock at -20°C for up to 1 year. |
| Reaction Buffer (Component A) | Add the corresponding azide or alkyne detection reagent directly to Reaction Buffer (Component A) for a final concentration of 40 μM. Store unused stock at -20°C. Stable for 1 year when stored as directed. |
| Copper (II) Sulfate (Component B) | Ready to use. Stable for 1 year when stored at 2-8°C |
| Reducing Agent (Component C) | Dissolve Reducing Agent (Component C) in 600 μL of deionized water, vortex vigorously for 2 minutes or until pellet is completely dissolved. Store unused stock at -20°C. Stable for 1 year when stored as directed. Note: reducing agent is susceptible to oxidation and turns brown when oxidized. If solution appears brown do not use. |
| Additive 1 (Component D) | Dissolve Additive 1 (Component D) in 600 μL of deionized water, vortex until completely dissolved. Store unused stock at -20°C. Stable for 1 year when stored as directed. |
1. Click Labeling Reaction
- For each azide or alkyne-modified protein lysate sample, add the following to a 1.5 mL microfuge tube, then vortex briefly to mix.
• Up to 50 μL protein lysate (1-5 mg/mL) in 50 mM Tris-HCl, pH 8.0, containing up to 1% SDS.
• 100μL Reaction Buffer (Component A).
• Cap the tube and vortex briefly to mix. - Add 10μL Copper (II) Sulfate (Component B), vortex briefly to mix.
- Add 10 μL Reducing Agent (Component C) to initiate click reaction, vortex briefly to mix. Wait for not longer than 5 minutes, before proceeding to the next step.
- Add 20 μL Additive 1 (Component D), vortex the tube briefly to mix. This solution turns bright orange.
- Protect reaction from light and vortex continuously or rotate end-over-end for 20-30 minutes at room temperature.
- Proteins in lysate are now click labeled and ready for downstream processing and/or analysis.
2. Preparation of Samples for Gel Analysis
- Add 600 μL methanol to 200 μL reaction mixture, vortex briefly.
- Add 150 μL chloroform, vortex briefly.
- Add 400 μL dH2O, vortex briefly.
- Centrifuge for 5 minutes at 13,000-20,000 x g, carefully remove upper aqueous layer without disturbing interface layer containing proteins.
Note: The upper aqueous layer is bright orange or contain color depending on detection reagent. - Add 450 μL methanol, vortex briefly.
- Centrifuge for 5 minutes at 13,000-20,000 x g to pellet protein. Carefully, remove and discard supernatant.
- Add 450 μL methanol, vortex briefly. Repeat step 6.
- Open the lid to microfuge tube and allow protein pellet to air-dry for at least 15 minutes.
- Cap and store labeled sample at -20°C until ready for use.
3. Gel Analysis
- Add50 μL gel electrophoresis sample buffer to protein pellet and vortex for 10 minutes to resuspend. Protein concentration will generally range from 1-5 μg/μL depending on starting protein concentration.
- Briefly spin protein/sample buffer mix before loading to remove any material that may not have been completely resuspended. Recommended loading amount for 1 -dimensional mini-gels is 5-20 μg.
- For SDS-PAGE gels, heat sample 10 minutes at 70°C prior to loading. For 2-dimensional gels resuspend in sample buffer, vortex for 10 minutes. Heat at 37°C for 10 minutes, if necessary.
- Perform electrophoresis as directed by manufacturer.
- Scan and image the gel immediately after removing gel from cassette.

